Introduction
On December 12, 2022, HHS released its notice of benefit and payment parameters for 2024 pertaining to the Affordable Care Act. This notice laid out several changes to the ACA program which would have significant impacts on all participants in the Marketplace ecosystem. After analyzing these changes, particularly those relating to the risk adjustment data validation (RADV) methodology, RaLytics formulated a comment letter, which we submitted to the HHS secretary during the public comment period. In it, we laid out several suggestions which we feel would improve the RADV process for all ACA stakeholders.
We are happy to share that letter below:
Re: Patient Protection and Affordable Care Act, HHS Notice of Benefit and Payment Parameters for 2024
Dear Secretary Xavier Becerra,
RaLytics™ LLC is pleased to submit comments on the proposed rule (CMS-9899-P]), Patient Protection and Affordable Care Act (ACA), Department of Health and Human Services (HHS) Notice of Benefit and Payment Parameters for 2024, specifically on the risk adjustment data validation (RADV) methodology.
RaLytics™ LLC is a certified Service-Disabled Veteran-Owned Small Business (SDVOSB) and leading Healthcare Information Technology (HIT) and data analytics company. We have extensive experience with RADV in the Medicare Advantage and commercial sectors, including for health plans participating in ACA Marketplaces. Our comments are based on our experience working with industry stakeholders in pursuit of a more efficient and equitable RADV process. Our comments can be summarized as follows:
- Equal access to quality medical record documentation across issuers is unlikely. The effectiveness of RADV and subsequent accuracy of the transfer payment is dependent on issuers having equal access to quality medical record documentation. However, access is a function of factors that vary by issuer, including each issuer’s business model, provider landscape, market leverage, and member HCC distribution.
- Unequal access for one issuer can impact transfer payments for all issuers in the market. The HHS RA program involves a zero-sum framework. Consequently, medical record inaccuracies for one issuer can significantly affect the transfer payment of other issuers in the market. Asymmetry in issuer ability to favorably measure their risk ultimately undermines the transfer payment’s objective—to accurately equilibrate risk among issuers within a market.
- Supplementing the RADV process with claim-based information can mitigate current shortfalls. The presence of health conditions can be evidenced by drugs, labs, and services indicated on claims submitted to the issuer for reimbursement. The development of predictive models using data submitted on claims to validate conditions would have the following benefits relative to RADV relying solely on medical record documentation:
- Reducing the burden of the RADV process. Acquisition of medical record documentation would be limited to only those HCCs that failed to meet the probability thresholds.
- Leveling the playing field for issuers. All issuers have equal access to claims data that could be used to identify and validate conditions.
- Improving transfer payment accuracy in equilibrating risk among issuers in a market. This would be achieved by reducing the asymmetry in the issuer’s ability to favorably measure their risk.
More details and analytical approach supporting these comments are provided below.
Background
Under the ACA, Qualified Health Plans (QHPs) or issuers are limited in the factors they are allowed to use in underwriting premiums. To prevent adverse selection, the statute and regulation1 authorize the use of risk adjustment to equilibrate risk within markets and mitigate the incentives for adverse selection. Based on plan-level risk relative to the risk faced by other plans in the market (“market risk”), the government facilitates the transfer of funds from issuers at lower risk relative to risk in the market to issuers at higher risk relative to the market risk.
RADV intends to ensure risk scores accurately reflect issuers risk by validating that diagnoses underlying risk scores are supported by medical record documentation. The effectiveness of the validation and subsequent accuracy of the transfer payment is thus dependent on the issuers equal access to quality medical record documentation. As that access is a function of the issuer’s business model, provider landscape, market leverage, member hierarchical conditions category (HCC) distribution, and other factors that vary by issuer, equal access to quality medical record documentation is unlikely. The purpose of this paper is to propose an alternative approach to validating risk scores with reduced reliance on medical record documentation.
Issue
The HHS-RADV process was borrowed from the Medicare Advantage RADV (MA-RADV) approach. While the basic mechanics of risk adjustment between the two programs are similar, the context and goals are different. MA-RADV’s objective is to ensure proper payment to Medicare Advantage Organizations (MAOs) from the government in a non-zero-sum framework. Variance in access to documentation would influence relative under or overpayment of a particular MAO without affect to the payment of any other MAO. In contrast, HHS-RADV’s objective is to ensure that risk scores used to calibrate the transfer payment equilibrating risk between issuers accurately reflect the issuers’ risk. Because HHS risk adjustment is in a zero-sum framework, inaccuracies in one issuer’s transfer payment can affect the transfer payment of other issuers in the market.
As discussed, the effectiveness of RADV is dependent on equal access to quality medical record documentation. An issuer’s access and influence over medical record documentation can positively affect their transfer payment by supporting disproportionate addition of diagnoses to the supplemental file and more completely supporting all diagnoses sampled for IVA audit. Issuers with greater market power or greater control over their submitting providers can create an advantage in the transfer payment to the detriment of other issuers in the market. The asymmetry in issuer ability to favorably measure their risk ultimately undermines the transfer payment’s objective of accurately equilibrating risk among issuers within a market.
Proposal
Given the vulnerabilities attributed to a RADV process solely dependent on medical record documentation, we propose an alternative approach to validate conditions included in the risk score. The presence of health conditions is also evidenced by the drugs, labs, and services indicated on claims submitted to the issuer for reimbursement. The development of predictive models using data submitted on claims to validate conditions would mitigate many of the vulnerabilities attributable to sole reliance on medical record documentation.
As a first step, we would propose using a predictive modeling approach as a filter on the current RADV sample. Probabilities below thresholds on each sampled HCC would be referred to the traditional RADV process for audit review. This approach would bring the immediate benefit of reducing the burden of the RADV process. Acquisition of medical record documentation would be limited to only those HCCs that failed to meet the probability thresholds. Second, all issuers have equal access to claims data leveling the playing field on identifying conditions for the supplemental file and validation of edge diagnoses in the sample satisfying the probability threshold. Finally, reducing the asymmetry in issuer ability to favorably measure their risk ultimately would improve the transfer payment accuracy in equilibrating risk among issuers within a market.
The proposed approach requires a predictive model to determine whether a member’s HCCs are substantiated through care patterns. This type of model can be trained on existing External Data Gathering Environment (EDGE) submissions used for risk adjustment.
We propose training a logistic regression model which uses indicators for procedures and prescriptions as its input and predicts each HCC in the HHS-HCC model as its output. The procedures are available directly from EDGE data through Healthcare Common Procedure Coding System (HCPCS) codes. Prescription data is available in EDGE as National Drug Code (NDC) codes, but we recommend converting to RxNorm codes to normalize prescription drugs to a more manageable volume of codes.
Model fit can be evaluated and directly measured by using a hold-out test sample from the EDGE data. A high precision at some threshold indicates whether there are strong claims-based indicators for an HCC and the recall at the threshold indicates how often those indicators are present. The presence of the HCCs for model testing could also be validated against existing prior-year audit data.
It is likely that the claims-based data would be most useful for many, but not all the HCCs (either because of small sample sizes to validate the model or HCCs that may be more likely to involve ambiguous clinical pathways). Thresholds should be used to balance accuracy of validation and number of HCCs that can be validated through this process.
Analytic Approach
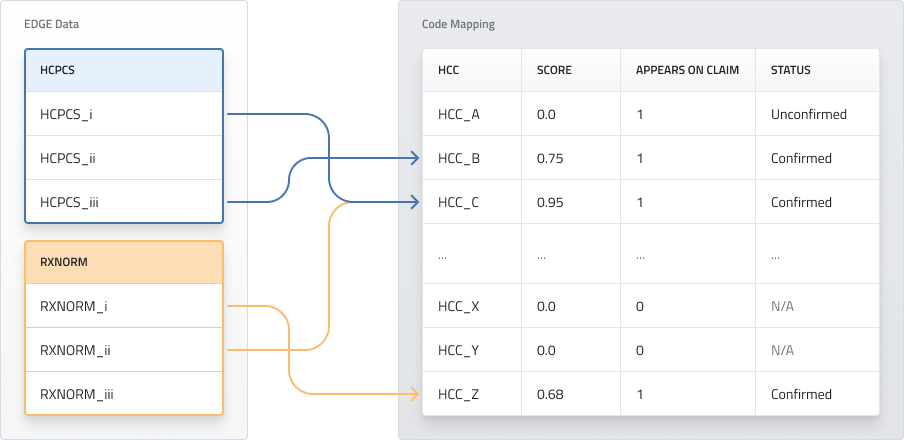
Figure 1: Illustration of analytic approach
Figure 1 shows an illustration of the proposed analysis. In this example HCPCS_i and RXNORM_ii are indicators for HCC_C, HCPCS_iii indicates HCC_B, and RXNORM_i indicates HCC_Z. The Score column measures how strongly the HCC is indicated overall and may be used to determine whether an HCC is validated. In this case, HCC_A appears on a claim but has no indicators of the conditions in the claim, giving it an “Unconfirmed” status, while HCC_B, HCC_C, and HCC_Z are confirmed by high scores. As a result, the necessity to acquire medical record documentation would be limited to HCC_X and HCC_Y.
We have done preliminary analysis to test this methodology and have identified HCCs that have strong claims-based indicators. These HCCs are typically associated with medical conditions which have strongly linked procedures (e.g., Pregnancy-related HCCs, Transplant-related HCCs) or prescription-drug use (e.g., HIV/AIDS). Expanding this analysis could provide a viable alternative method for confirming HCCs from claims data.
Conclusion
As HHS reviews and considers comments to your proposals regarding RADV, the priority should be to create a more efficient system that allows health plans to focus on providing their members high-quality health care while limiting potential biases against certain types of plans. As the landscape of plans offered on the State Marketplaces continues to evolve, it is critical to maintain the competitive nature that has developed over time and ideally will continue to offer multiple options of viable health plans to consumers in all States.
Thank you for the opportunity to provide input on this important matter.
Respectfully submitted,
William J. Dawkins
Principal